II. Viruses
III. Coronaviruses
IV. Citations
I. About This Primer
Without a doubt, the biggest story of 2020 is the coronavirus epidemic. 1 By the middle of the year, I, like many others out there, felt overwhelmed in a muddle of fragmented news and science, misinformation, and general confusion. I announced on Facebook that I wanted to “digest it all” and assemble the best known information into one primer. “Any questions you’d like me to research?” I asked, and a few friends immediately chimed in with requests. Hopefully, this will help us understand coronavirus basics from the ground up. I will begin this primer with the big picture and then gradually zoom in from viruses to coronaviruses to this year’s unwanted pests. There is far too much ground to cover in one article, so I’m breaking it into multiple parts. Today’s post is Part 1: Background. This article discusses viruses and coronaviruses in general.
II. Viruses
A. Square One: What is a Virus, Anyway?
B. Infection, Immunity, Inoculation
A. Square One: What is a Virus, Anyway?
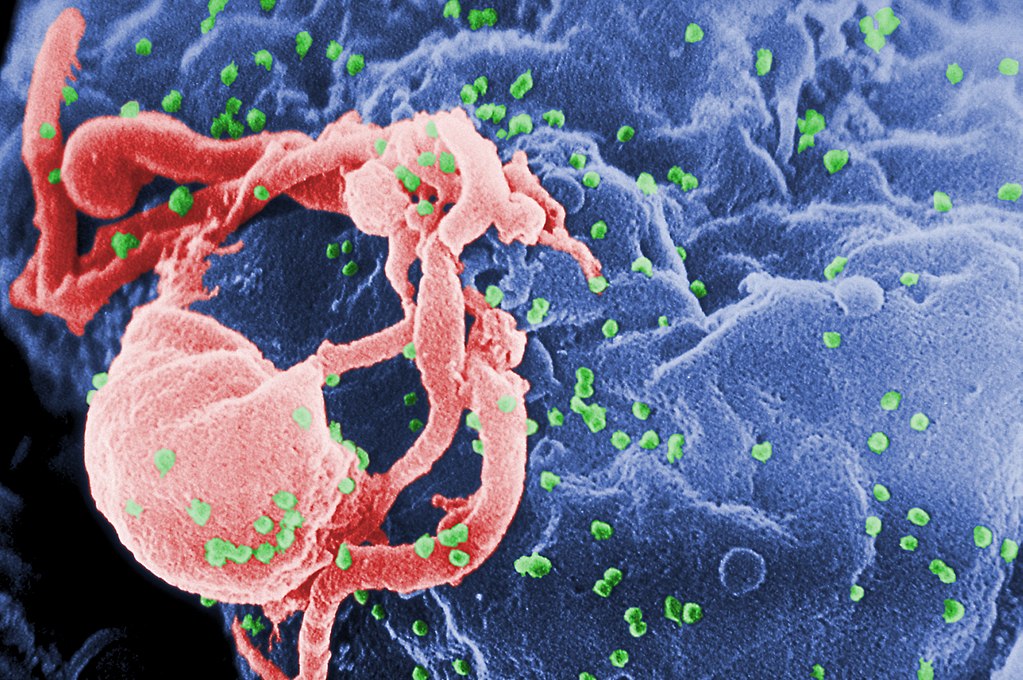
Viruses occupy one of the most intriguing positions in the whole grand scheme of things. They are microbes right at the boundary of life and non-living chemistry. This makes them primary subjects of interest for scientists studying the origins of life.
You are made of about 30 trillion cells. 2 We call a cell the smallest unit of life because it performs all the functions that we expect of a living thing. A cell grows, responds to stimuli, metabolizes, heals, sustains and defends itself, reproduces, and dies. However, it is not a unitary blob of spittle. A cell is a complex system with hundreds of interacting parts, with names like macromolecules and organelles. Most of those parts specialize in just one life function, so by themselves they are not fully alive. If I could compare a virus to anything else, I’d say it’s like a cell organelle that specializes in reproduction.
Viruses are like living things in numerous ways. They are made of the same basic macromolecules as us: nucleic acids, proteins, lipids, and carbohydrates. At the core of every virus is a bundle of DNA or RNA comprising a few genes. Viruses reproduce and evolve / speciate. Biologists classify them and give them Latinesque scientific names. Viruses can be “killed” by heat, chemicals, or radiation.
However, viruses are like lifeless chemicals in that they don’t metabolize, respond, heal, change, or grow. Nor are they able to protect themselves from the environment. And although viruses specialize in just one thing – reproduction – they can’t even do that by themselves. A virus is an absolute parasite. In order to function, it must inhabit a living cell. The “life” cycle of a virus is to invade a cell and exploit the cell’s resources to make dozens of copies of itself, which then burst forth to invade other cells. Viruses infect the cells of all living things, from bacteria to whales. They kill more life forms, and more humans, than any other force of nature.
B. Infection, Immunity, Inoculation
While some viruses are harmless or even beneficial, most of them cause irreparable or fatal damage to their host cells. Rapidly reproducing viruses consume a cell completely and then burst out like a battalion of creepy little microscopic robots. Since they are vulnerable outside of cells, they must pass quickly from dying cell to living cell. They can pass from one person to another through skin, body fluids, or air currents.
Viral infection involves proteins on the surfaces of viruses and cells. Proteins have complex three-dimensional shapes like locks and keys. If a virus has the protein “key” to a cell’s outer “lock”, it will latch on and inject its genes inside.
In a human or animal body, fragments of viruses called antigens react with our white blood cells, aka our immune system. In lucky cases, the immune system produces a protein called an antibody, a natural defense. An antibody latches onto the antigen that stimulated it. Sometimes, the antibody disfigures the virus’s “key” so that the virus can no longer penetrate cells. Other antibodies “flag” a virus so that white blood cells can easily identify and destroy it. While a person has an antibody in his blood, he is immune to that virus. The next time the virus comes along, his antibodies will latch onto the virus’s antigens and slow it down or stop it. Some antibodies last a lifetime, while others disappear from the bloodstream in a few years. A vaccine is a human-made, finely tuned dose of antigens – just enough to stimulate an immune response without a full-blown infection. If enough people in a community become immune to the virus, whether through natural immunity or vaccine inoculation, the virus begins to die out and can even go extinct.
It’s important to note that viruses evolve quickly. This is especially true of RNA viruses, which most human viruses are. When a virus evolves, its proteins can change shape. That is a headache for us; we are forced to keep reinventing new locks to morphing viral keys. Occasionally, a virus that infects one animal will evolve to a new form that infects another animal, including humans. A virus that jumps ship from one species of host to another is called zoonotic.
C. Human Viral Diseases
A viral disease is not quite the same as the virus that causes it. A disease is the physical manifestation of the virus, its effects on the person. Sometimes a virus and its disease have different names, as HIV (Human Immunodeficiency Virus) causes AIDS (Acquired Immuno-Deficiency Syndrome). In other cases, we use a single term, like “Ebola”, to name both the virus and its disease. There are too many human disease-causing viruses to list here. Examples include adenoviruses, astroviruses, encephalitis, enteroviruses, hepatitis, herpes, HPV, influenza, measles, meningitis, mumps, noroviruses, parainfluenza, polio, pox, rabies, rhinoviruses (which cause colds), roseola, rotaviruses, rubella, West Nile, Zika, … you get the picture.
D. Measuring Epidemics
Viruses are inherently public health threats. Human viruses would quickly die out if they did not have lots and lots of people to infect. Viral infections can be quantified in numerous ways. The three most fundamental independent metrics are reproduction number, transmission time, and case-fatality rate.
Reproduction number is commonly abbreviated as R0. It measures the average number of healthy persons who catch the virus from each infected person. For example, if each sick person makes four other people sick, then R0 = 4.
Transmission time is the average time it takes a virus to spread from one person to another. I don’t see this factor discussed very often, but it makes a big difference whether a sick person infects others in a matter of hours (like an airborne virus in a mall) or years (like a sexually transmitted virus).
Finally, the infection-fatality rate is the most morbid statistic of them all: the percentage of infected persons who die. The number of serious illnesses or hospitalizations may be measured as well; they will be closely correlated to the fatality rate.
Unfortunately, the fundamental metrics above are difficult to measure. We can’t measure them all in a lab because they are not entirely intrinsic to the virus. They depend on human activity too. It’s hard to know how many people are infected when not everyone is tested and / or exhibiting symptoms.
The easiest way to measure the severity of an epidemic is with the number of deaths and / or hospitalizations. These numbers are documented well. There will still always be some over-reporting (deaths attributed to the virus that were really due to something else) and under-reporting (deaths due to the virus that weren’t counted). On a societal level, sheer numbers are more meaningful than rates. It doesn’t really matter if there are 1,000 infections that are 10% fatal or 10,000 infections that are 1% fatal. Both scenarios will result in 100 deaths and should be considered equally dangerous. Of course, if you get infected, you sure will be interested in knowing if you have a 1% or 10% chance of dying!
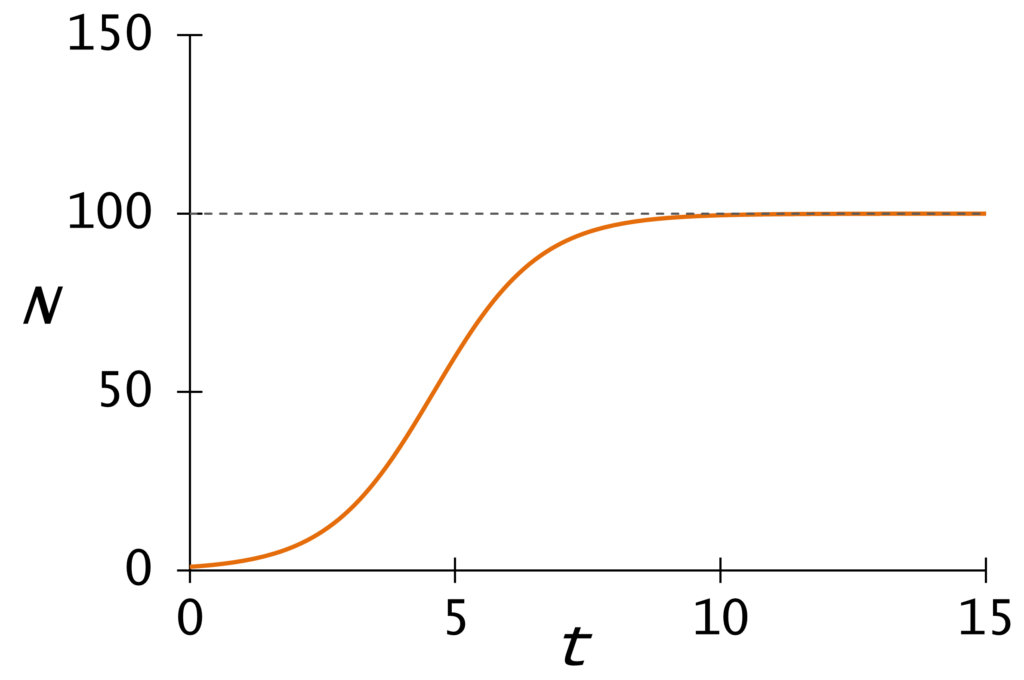
Taken together, the reproduction number and transmission time determine the doubling time, or the amount of time it takes for the prevalence of infections to double in the population. The pattern characterized by a constant doubling time is called exponential growth. In real life, exponential growth never lasts long, because it runs up against limitations like immunity or finite populations. Doubling time is the metric we hear most on the news, because it is easy to calculate by simply counting cases. Breaking it down offers slightly more insight, if only theoretically. When doubling time increases, it demonstrates that each sick person is infecting fewer healthy people and / or she’s doing so more slowly, which are the intended effects of social distancing.
When doubling time slows down (as it always must) the simplest model for epidemic growth is the logistic formula. This S-shaped curve represents the all-time number of cases “flattening” as it reaches its maximum and stops growing. The point where the number of daily cases starts to decrease is the point of inflection. I hear some people using the term “inflection point” as if it means the point where a virus “really takes off”. That’s the opposite of the correct meaning, and there is no well-defined point of acceleration on the curve.
Real epidemiologists use sophisticated numerical algorithms that model the interactions among Susceptible, Immune, and Recovered people. These SIR or compartmentalized models are run on supercomputers.
E. Treatment Options
Besides vaccines, there are at least two medical options for some viral infections.
Antiviral drugs kill viruses after infection. Antiviral pharmacology is recent technology. It is more sophisticated than vaccinations. To produce an antiviral medicine, researchers sequence the virus’s RNA and proteins and then engineer molecules to target the virus’s vulnerable points. This highly advanced research developed in response to the AIDS pandemic.
Each vaccine or antiviral medication is engineered specifically for one strain of virus. Though the vaccines for polio and smallpox were “miracle cures” that drove their viruses to extinction, they are useless against other viruses. Medical labs are experienced and pretty effective at making influenza vaccines. On the other hand, centuries of effort have still yielded no rhinovirus vaccines.
Because vaccines and antivirals are not always available, hospitals must also rely on symptomatic treatments, which only mitigate the disease without managing the virus. Some drugs treat symptoms such as inflammation (also known as cytokine storm). In extreme cases, medical equipment like ventilators assist with breathing while the virus passes.
III. Coronaviruses
B. The First Two Killer Coronaviruses
A. What Coronaviruses Are
Coronaviruses are classified as the family Coronaviridae in the virus family tree. They get their name from the “spike” proteins, also known as S proteins, embedded in their fatty envelope. The spikes look like the corona (crown) of the sun, and they are the killer proteins involved in latching onto host cell membranes.
Coronaviruses have infected bats and birds for tens or hundreds of millions of years. 4 They are occasionally transmitted to other mammalian species that come into contact with bats. There are now five genera of coronaviruses. Two of them, Alphacoronavirus and Betacoronavirus, include species that infect humans. Today’s living alpha- and beta-coronaviruses descend from a common ancestor four or five millennia ago. 5
Some animal diseases that are now recognized as coronavirus infections came to veterinary attention in the early 20th century. The viruses themselves were only observed and named in the 1960s. Since then, the medical community has identified seven coronaviruses that infect humans. Four of them only cause colds. The three most recent strains, all beta-coronaviruses that evolved in the 21st century, are much more severe. There are no known treatments for any of them.
B. The First Two Killer Coronaviruses
The first alarming coronavirus outbreak was the SARS epidemic of 2002 – ’04. SARS stands for Severe Acute Respiratory Syndrome. 2 The virus that caused it was named SARS-CoV. The SARS coronavirus originated in horseshoe bats in southern China. 6 It was then apparently transmitted to intermediary species that were sold in exotic animal markets: the palm civet (a wild cat), the raccoon dog (a wild dog), and / or the ferret badger. All of these species carried SARS-CoV-like viruses. The exact pathway from bat to carrier to human has not yet been solved.
SARS had a high case-fatality rate, about 10%. It was easy to detect infected people, though, because almost everybody who caught it broke out into fever and coughs within 2 – 3 days. The sick were quickly quarantined and questioned about their most recent contacts, who were also isolated. Local governments also ordered a mass killing of palm civets. 7 Even without a vaccine or anti-viral remedy, the anti-SARS campaign was a complete success. Only 8,000 people ever caught this disease. Canada was the only non-Asian country with more than one death. Curiously, just like COVID-19, SARS barely touched Africa. The peak of the outbreak lasted just a few months, February – July 2003. The onset of summer weather slowed it down. By 2004, SARS-CoV was extinct.
Living in a world city, Los Angeles, I have long noticed Chinese nationals wearing face masks all the time, and I always wondered why. It’s the SARS outbreak that got them in the habit, and many have worn masks routinely ever since 2003. It doesn’t seem so unusual anymore!
The next major coronavirus outbreak was called MERS (Middle East Respiratory Syndrome) because it was concentrated in Saudi Arabia and neighboring countries. The MERS virus passed from bats to camels in the 1990s and then to people who made close contact with camels in 2012. This virus had a distinct profile. MERS had a much higher case-fatality ratio. 30 – 35% of the patients who caught it died! Fortunately, it did not easily pass from one person to another. The MERS virus still exists, but it has only killed 900 people, just a few each year now.
In 2017, the scientists who traced SARS-CoV to a bat cave in Yunnan Province observed that the viruses were recombining (intermixing) to form myriad new combinations. Their discussion included this prophetic warning:
“We have also revealed that various SARSr-CoVs … are still circulating among bats in this region. Thus, the risk of spillover into people and emergence of a disease similar to SARS is possible.” 8
Ben Hu (2017)
Continue to Coronavirus Primer Part 2: SARS-CoV-2, COVID-19, and the Individual
IV. Citations
- Photo Credit: C. GoldsmithContent Providers: CDC/ C. Goldsmith, P. Feorino, E. L. Palmer, W. R. McManus / Public domain. https://commons.wikimedia.org/wiki/File:HIV-budding-Color.jpg (accessed and saved 6/23/20). ↩
- Ron Sender, Shai Fuchs, and Ron Milo, “Revised Estimates for the Number of Human and Bacteria Cells in the Body”, PLOS Biology (8/19/2016), https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1002533 (accessed 7/06/20). ↩
- Logistic curve by Yapparina / CC0, https://commons.wikimedia.org/wiki/File:Logistic_curve,_r%3D1,_K%3D100,_N0%3D1.png (accessed 7/07/20). ↩
- Joel O. Wertheim et al., “A Case for the Ancient Origin of Coronaviruses”, Journal of Virology 87(12):7039-45 (June, 2013), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3676139/ (accessed and saved 6/25/20). ↩
- Patrick C.Y. Woo et al., “Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus”, Journal of Virology 86(7):3995-4008 (Apr., 2012), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3302495/ (accessed and saved 6/25/20). ↩
- Ben Hu et al., “Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insight into the origin of SARS coronavirus”, PLOS Pathogens 13(11):e1006698 (11/30/2017), https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006698 (accessed and saved 6/25/20). ↩
- Jane Parry, “WHO queries culling of civet cats”, BMJ 328(7432):128 (1/17/2004), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1150312/ (accessed and saved 7/03/20). ↩
- Hu (2017), op. cit., “Discussion”, last paragraph. ↩

